
What are Peptides?
Peptides are short chains of amino acids joined by peptide bonds.
They’re vital to well-being because amino acids are the core components of life, like tiles in a mosaic.
With just 20 amino acids, our body can build all the proteins it needs to thrive.

When a small group of these amino acids—typically 2 to 50—connect, they form a peptide. More than 50 is a protein.
Think of amino acids as individual puzzle pieces.
Peptides are like small, completed sections of the puzzle, while proteins are the entire picture.
Just as puzzle sections create distinct parts of an image, peptides carry out specific tasks in the body.

Peptides’ value comes from their ideal balance of size and accuracy.
They’re large enough to deliver precise biological instructions and connect with specific cellular targets, yet small enough to be absorbed quickly and travel efficiently.
This “perfect-fit” trait makes peptides excellent coordinators of bodily processes.

Our Natural Peptides
Our body produces thousands of distinct peptides, each with specialized roles.
These internally created (endogenous) peptides perform various functions.
• Neural messengers: Some peptides act as signals in the nervous system, helping brain cells communicate.
• Hormones: A few examples are – ghrelin, which regulates appetite – parathyroid hormone, which supports bone strength, and relaxin, linked to tissue flexibility.
• Digestive assistants: Peptides in the stomach help break food into nutrients the body can absorb.
• Immune protectors: Peptides like hepcidin defend against pathogens such as bacteria, viruses, and fungi, reinforcing your body’s defenses.
• Cellular directors: Many peptides guide cells to grow, split, produce specific proteins, or undergo natural turnover, a key part of tissue health.
•••
These peptides work with pinpoint precision, latching onto specific cell receptors somewhat like magna tiles.

This accuracy enables the body to manage its complex systems seamlessly.
NEW – Consider our AI Tool!
Lab-Created Peptides
In addition to natural peptides, scientists can create peptides in labs, opening new doors for health and treatment.
These lab-made peptides can:
1. Imitate natural peptides: Some synthetic peptides closely match those the body produces, boosting its natural reserves.
2. Improve natural peptides: Researchers can tweak peptides to enhance their stability or strengthen cellular connections.
3. Develop new peptides: Scientists can also create new peptides that don’t exist in nature but can interact with biological systems in helpful ways.
The ability to design and refine peptides has revolutionized healthcare, offering precise solutions with:
- greater effectiveness than other natural options (see our post here for a comparison to herbs)
- fewer side effects and in many cases better results than standard drugs
From aiding recovery to treating specific conditions, peptides provide customized benefits.
How Peptides Interact with Cells
The Magna Tile analogy
Most peptides operate through a “receptor-attachment” process.
Picture a cell as if the surface was dotted with metallic pieces (receptors).
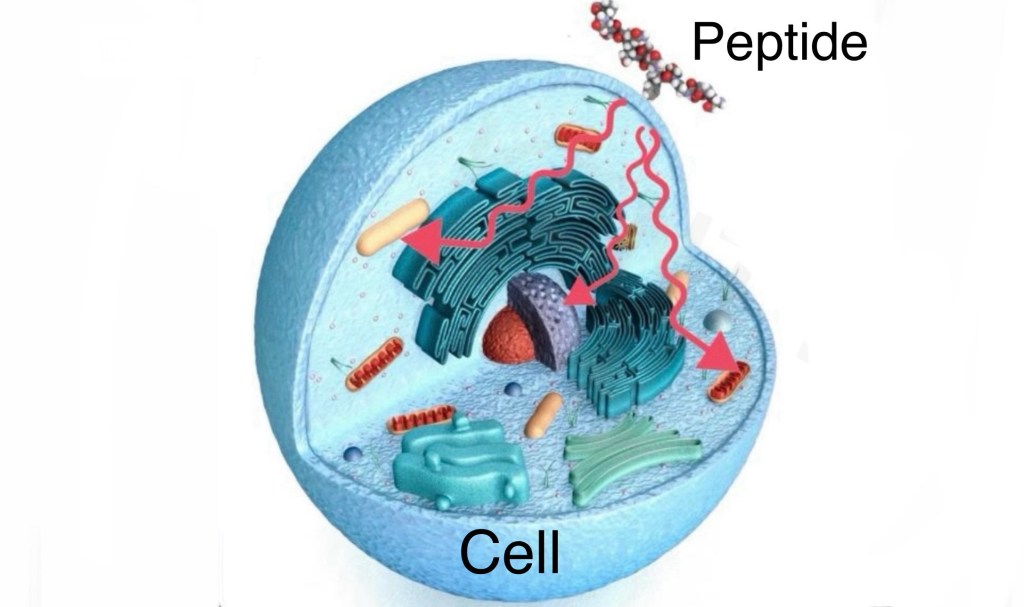
Specific peptides perform like magnets that attach to the metal parts precisely.

When a peptide connects, it sets off a series of cellular actions, like flipping on a switch.
This process, called signal transmission, allows peptides to send instructions to cells.
Their precision is impeccable. They attach only to specific receptors that target particular cells or tissues.
This focus reduces unwanted effects, making peptides highly effective with minimal side effects.
Types of Peptide-Cell Connections
Peptides interact with different receptor types, each triggering unique cellular responses.
1. Gene-regulating: Certain peptides influence DNA activity by connecting with receptors inside the cell’s core.
2. G-protein-linked: Many peptide hormones and signals use these to start cellular processes.
3. Ion-flow: Some peptides affect pathways that control ion movement, altering cell behavior.
4. Enzyme-activated: Enzymes can change cell actions, encouraging growth or specialization.
The receptor type shapes the peptide’s impact on the cell.

What Happens After Connection?
When a peptide attaches to a cell receptor, it triggers a “signaling chain,” leading to key effects such as:
- Turning genes on or off to create new proteins.
- Prompting cells to grow, divide, release substances, or undergo natural death.
- Adjusting how cells metabolize, process energy or materials.
- Changing sensitivity and how cells respond to other signals.

These changes lead to noticeable effects, such as:
• Quicker healing with tissue-repair peptides such as TB4-Frag.
• Reduced inflammation and natural elimination of bacteria and fungi with Antimicrobial / antiinflammatory peptides, such as KPV.
• Enhanced thyroid performance with thyroid peptides, such as ThyroPep.
• Stronger immunity with immune modulating peptides such as Thymogen.

How Peptides Move Through the Body
After being introduced via oral capsules, sublingually, as nasal sprays, injections or topically, peptides:
Spread throughout the body, with many (especially bioregulator peptides) designed to focus on specific areas.
Attach to receptors in cells in target tissues, signaling them to produce effects.
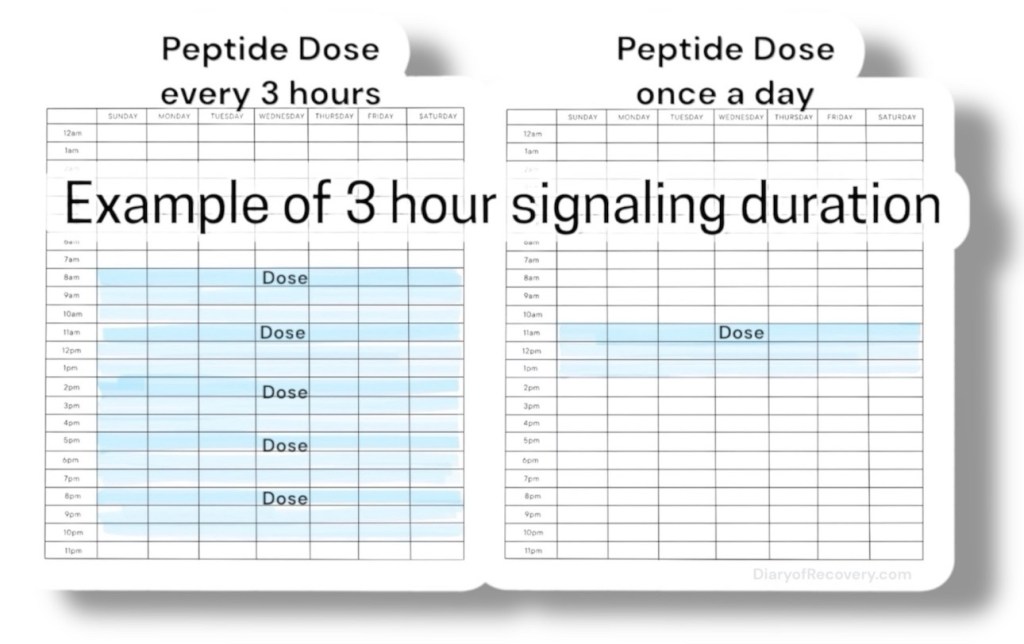
This signaling duration is short lived and lasts only minutes to hours at most (often requiring frequent dosing for the first few weeks of taking them).
After taking them for a longer time, epigenetic changes can occur, and less frequent dosing is needed

Finally, they are broken down into amino acids by enzymes called peptidases, filtered by the kidneys and excreted.
The end!
More from us:

- Blogs page
- Facebook Group
Extra Credit – The History of Peptides

The story of peptide therapy reflects scientific progress, growing from early insights into a cutting-edge medical field.
Peptide therapy’s roots trace back to the early 20th century, when researchers began extracting hormones from animal tissues.
In 1922 they isolated insulin, a peptide hormone critical for managing blood sugar, from animal sources.
By 1924, insulin was widely available, transforming diabetes treatment and marking a milestone in peptide use.
Through the mid-20th century, scientists uncovered other peptides, like oxytocin, somatotropin, and corticotropin, exploring their medical potential.
The Synthetic Shift
A game-changer occurred in 1954 when vasopressin was created — the first synthetic peptide hormone.
It proved peptides could be manufactured, expanding their therapeutic possibilities.
By the 1970s and 1980s, techniques like automated peptide synthesis streamlined production, allowed for more complex peptides to be made efficiently.
Today’s Peptide Innovations
Peptide therapies are now a critical part of healthcare.
Over 70 peptide drugs are approved with many more in testing.
Their uses cover a wide range:
• Hormone health: Peptides like ghrelin analogs or relaxin for hormone balance.
• Cancer care: Peptides that target tumors or boost immune defenses against cancer.
• Brain health: Peptides supporting mental clarity and neural protection.
• Skin care: Peptides promoting skin repair and moisture retention.
• Athletic recovery: Peptides aiding muscle repair and performance.
• Aging support: Peptides tackling age-related changes.
Improvements in delivery, durability, and targeting have made peptide therapies more practical and effective.
From Experimental to Mainstay
Peptide therapy has moved from an experimental niche to a mainstay in healthcare over the past decade due to:
• Solid research proving peptide safety and effectiveness.
• Better training for doctors on peptide uses.
• Rising demand for tailored wellness solutions.
• Manufacturing advances making peptides more accessible.
While some uses remain cutting-edge, many peptide therapies are now backed by robust research and clinical experience, making them key tools in health optimization.
